Combating Striga
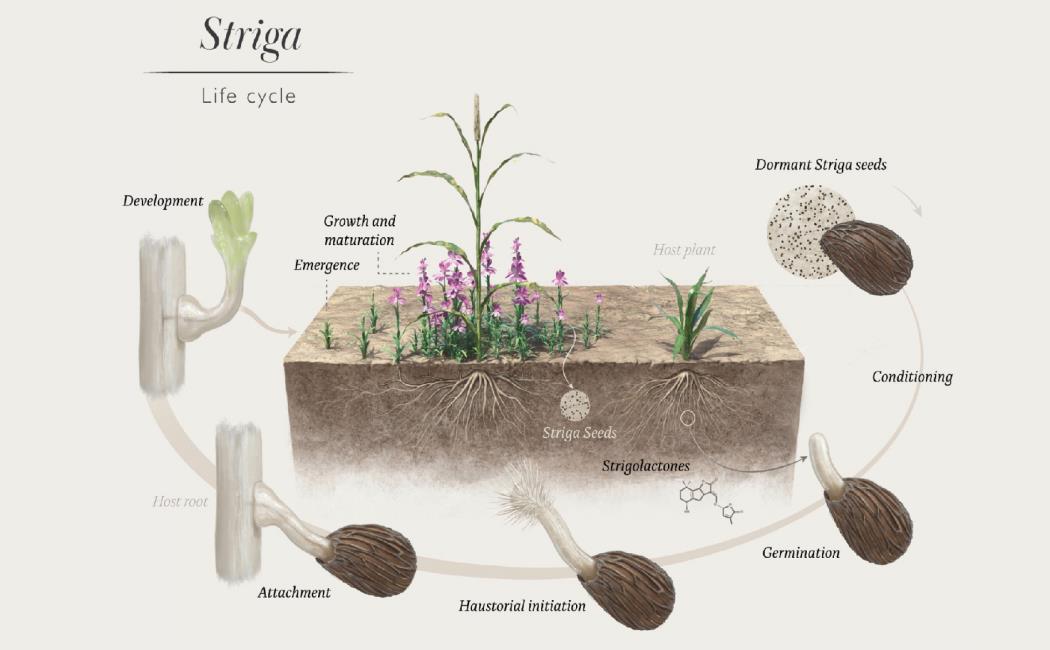
Root parasitic plants, such as the witch weed Striga spp. [S. hermonthica (Del.) Benth. and S. asiatica (L.) Kuntze] and the broomrape Orobanches of the Orobanchaceae family, are a major agricultural pest that is considered as one of the seven greatest biological constraints to food production, causing large yield losses in many crops including corn, millet, sorghum, legumes, rapeseed, and tomato. Striga and Orobanche seeds germinate only in the presence of host plants that release strigolactones into the soil, which are required as germination stimulants. Following germination, seedlings develop haustoria that connect them to the vascular tissues of the host and enable the uptake of photosynthetic products, minerals and water. This connection is essential for the survival of these obligate parasites that will then grow, bloom and set enormous numbers of seeds at the cost of the host plant. Deployment of control strategies that reduce the parasite seed bank and at the same time minimize host-plant root infection are likely to be the most promising control option. In this project, we aim at developing synthetic strigolactone-analogues that can be used to induce the so called “suicidal germination”, i.e. germination in the absence of host plants, and of novel-chemistries that reduce SL release and improve host growth. Moreover, we aim at identifying genetic factors determining SL pattern and employing them for increasing resistance by genetic engineering or through breeding. For more information, visit our webpage Striga solutions at https://strigasolutions.com/ .
- Prof. Tadao Asami, University of Tokyo, Japan
- Prof. Steven Runo, Kenyatta University, Kenya
- Dr. Djibril Yonli, INERA, Burkina Faso
- Dr. Dennis Tippe, TARI, Tanzania
- Patrick Mudavadi, KALRO, Kenya
- Dr. Prashant Kini, UPL-India
- Ablazov A, Votta C, Fiorilli V, Wang JY, Aljedaani F, Jamil M, Balakrishna A, Balestrini R, Liew KX, Rajan C (2023) ZAXINONE SYNTHASE 2 regulates growth and arbuscular mycorrhizal symbiosis in rice. Plant Physiology 191: 382-399
- Yang Y, Abuauf H, Song S, Wang JY, Alagoz Y, Moreno JC, Mi J, Ablazov A, Jamil M, Ali S (2023) The Arabidopsis D27‐LIKE1 is a cis/cis/trans‐β‐carotene isomerase that contributes to Strigolactone biosynthesis and negatively impacts ABA level. The Plant Journal
- Jamil M, Wang JY, Yonli D, Patil RH, Riyazaddin M, Gangashetty P, Berqdar L, Chen G-TE, Traore H, Margueritte O (2022) A New Formulation for Strigolactone Suicidal Germination Agents, Towards Successful Striga Management. Plants 11 (6):808
- Jamil M, Wang JY, Yonli D, Ota T, Berqdar L, Traore H, Margueritte O, Zwanenburg B, Asami T, Al-Babili S (2022) Striga hermonthica suicidal germination activity of potent strigolactone analogs: Evaluation from laboratory bioassays to field trials. Plants 11: 1045
- Wang JY, Braguy J, Chen GTE, Jamil M, Balakrishna A, Berqdar L, Al-Babili S (2022) Perspectives on the metabolism of strigolactone rhizospheric signals. Frontiers in Plant Science 13: 1-9
- Ito S, Braguy J, Wang JY, Yoda A, Fiorilli V, Takahashi I, Jamil M, Felemban A, Miyazaki S, Mazzarella T (2022) Canonical strigolactones are not the major determinant of tillering but important rhizospheric signals in rice. Science Advances 8: eadd1278
- Chen G-TE, Wang JY, Jamil M, Braguy J, Al-Babili S (2022) 9-cis-β-Apo-10ʹ-carotenal is the precursor of strigolactones in planta. Planta 256: 88-93.
- Wang JY, Jamil M, Hossain MG, Chen G-TE, Berqdar L, Ota T, Blilou I, Asami T, Al-Solimani SJ, Mousa MAA (2022) Evaluation of the Biostimulant Activity of Zaxinone Mimics (MiZax) in Crop Plants. Frontiers in Plant Science 13: 1-13.
- Xiao TT, Kirschner GK, Kountche BA, Jamil M, Savina M, Lube V, Mironova V, Al Babili S, Blilou I (2022) A PLETHORA/PIN-FORMED/auxin network mediates prehaustorium formation in the parasitic plant Striga hermonthica. Plant Physiology 189: 2281-2297
- Wang JY, Chen G-TE, Jamil M, Braguy J, Sioud S, Liew KX, Balakrishna A, Al-Babili S (2022) Protocol for characterizing strigolactones released by plant roots. STAR Protocols 3 (2):101352
- Zarban RAY, Hameed UFS, Jamil M, Ota T, Wang JY, Arold ST, Asami T, Al-Babili S (2021) Rational design of Striga hermonthica-specific seed germination inhibitors. Plant Physiology 192:1-10
- Wang JY, Alseekh S, Xiao T, Ablazov A, Perez de Souza L, Fiorilli V, Anggarani M, Lin P-Y, Votta C, Novero M, Jamil M, Lanfranco L, Hsing Y-IC, Blilou I, Fernie AR, Al-Babili S (2021) Multi-omics approaches explain the growth-promoting effect of the apocarotenoid growth regulator zaxinone in rice. Communications Biology 4 (1):1-11
- Braguy J, Ramazanova M, Giancola S, Jamil M, Kountche BA, Zarban RAY, Felemban A, Wang JY, Lin P-Y, Haider I (2021) SeedQuant: A deep learning-based tool for assessing stimulant and inhibitor activity on root parasitic seeds. Plant Physiology 186 (3):1632-1644
- Jamil M, Kountche BA, Al-Babili S (2021) Current progress in Striga management. Plant physiology 185 (4):1339-1352
- Wang JY, Jamil M, Lin P-Y, Ota T, Fiorilli V, Novero M, Zarban RA, Kountche BA, Takahashi I, Martínez C (2020) Efficient Mimics for Elucidating Zaxinone Biology and Promoting Agricultural Applications. Molecular Plant 13:1654-1661
- Ablazov A, Mi J, Jamil M, Jia K-P, Wang JY, Feng Q, Al-Babili S (2020) The apocarotenoid zaxinone is a positive regulator of strigolactone and abscisic acid biosynthesis in arabidopsis roots. Frontiers in Plant Science 11: 578-586
- Jamil M, Kountche BA, Wang JY, Haider I, Jia K-P, Takahashi I, Ota T, Asami T, Al-Babili S (2020) A new series of carlactonoic acid based strigolactone analogs for fundamental and applied research. Frontiers in Plant Science 11: 434-440
- Jamil M, Imran Haider, Boubacar A. Kountche, Al-Babili S (2019) Effect of D-ring C-3' methylation of strigolactone analogs on their transcription regulating activity in rice. Plant Signaling & Behvior 14: 12-15.
- Jamil M, Kountche BA, Haider I, Wang JY, Aldossary F, Zarban RA, Jia K-P, Yonli D, Hameed UFS, Takahashi I (2019) Methylation at the C-3′ in D-Ring of Strigolactone Analogs Reduces Biological Activity in Root Parasitic Plants and Rice. Frontiers in Plant Science 10:1-14.
- Wang JY, Haider I, Jamil M, Fiorilli V, Saito Y, Mi J, Baz L, Kountche BA, Jia K-P, Guo X (2019) The apocarotenoid metabolite zaxinone regulates growth and strigolactone biosynthesis in rice. Nature Communications 10:810-819.
- Kountche BA, Jamil M, Yonli D, Nikiema MP, Blanco‐Ania D, Asami T, Zwanenburg B, Al‐Babili S (2019) Suicidal germination as a control strategy for Striga hermonthica (Benth.) in smallholder farms of sub‐Saharan Africa. Plants, People, Planet 1 (2):107-118.
- Kountche BA, Novero M, Jamil M, Asami T, Bonfante P, Al-Babili S (2018) Effect of the strigolactone analogs methyl phenlactonoates on spore germination and root colonization of arbuscular mycorrhizal fungi. Heliyon 4 (11):e00936.
- Jamil M, Kountche BA, Haider I, Guo X, Ntui VO, Jia K-P, Ali S, Hameed US, Nakamura H, Lyu Y, Jiang K, Hirabayashi K, Tanokura M, Arold ST, Asami T, Al-Babili S (2018) Methyl phenlactonoates are efficient strigolactone analogs with simple structure. Journal of Experimental Botany 69:62319-62331.
- Butt H, Jamil M, Wang JY, Al-Babili S, Mahfouz M (2018) Engineering plant architecture via CRISPR/Cas9-mediated alteration of strigolactone biosynthesis. BMC Plant Biology 18 (1):174-182.
- Hameed US, Haider I, Jamil M, Kountche BA, Guo XR, Zarban RA, Kim D, Al-Babili S, Arold ST (2018) Structural basis for specific inhibition of the highly sensitive ShHTL7 receptor. EMBO Reports 19 (9).
- Baz L, Mori N, Mi JN, Jamil M, Kountche BA, Guo XJ, Balakrishna A, Jia KP, Vermathen M, Akiyama K, Al-Babili S (2018) 3-Hydroxycarlactone, a Novel Product of the Strigolactone Biosynthesis Core Pathway. Molecular Plant 11 (10):1312-1314.